| Mechanism | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | Ownership | PIPE-791 |
|---|---|---|---|---|---|---|---|---|
| LPA1R Antagonist | IPF* | Wholly-owned | ||||||
| close x | ||||||||
| LPA1R Antagonist | PPMS/SPMS* | Wholly-owned | ||||||
| close x | PIPE-307 | |||||||
| M1R Antagonist | RRMS | In Collaboration with Johnson & Johnson | ||||||
| close x | ||||||||
| M1R Antagonist | Depression | In Collaboration with Johnson & Johnson | ||||||
| close x | ||||||||
| LPA1R Antagonist
IPF* | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
Wholly-owned | ||||
| LPA1R Antagonist
PPMS/SPMS* | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
Wholly-owned | ||||
| M1R Antagonist
RRMS | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
In Collaboration with Johnson & Johnson | ||||
| M1R Antagonist
Depression | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
In Collaboration with Johnson & Johnson | ||||
* Partnered
**Wholly-owned
LPA1R Antagonist
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (Progressive MS). We are currently conducting a Phase 1 clinical trial of PIPE-791 in healthy volunteers for the potential treatment of IPF. Thereafter, we plan to commence Phase 1b open-label trials in each of IPF and Progressive MS to measure lung and brain receptor occupancy, respectively, by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
LPA1R Antagonist
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (Progressive MS). We are currently conducting a Phase 1 clinical trial of PIPE-791 in healthy volunteers for the potential treatment of IPF. Thereafter, we plan to commence Phase 1b open-label trials in each of IPF and Progressive MS to measure lung and brain receptor occupancy, respectively, by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
M1R Antagonist
PIPE-307 is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.
M1R Antagonist
PIPE-307 is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.
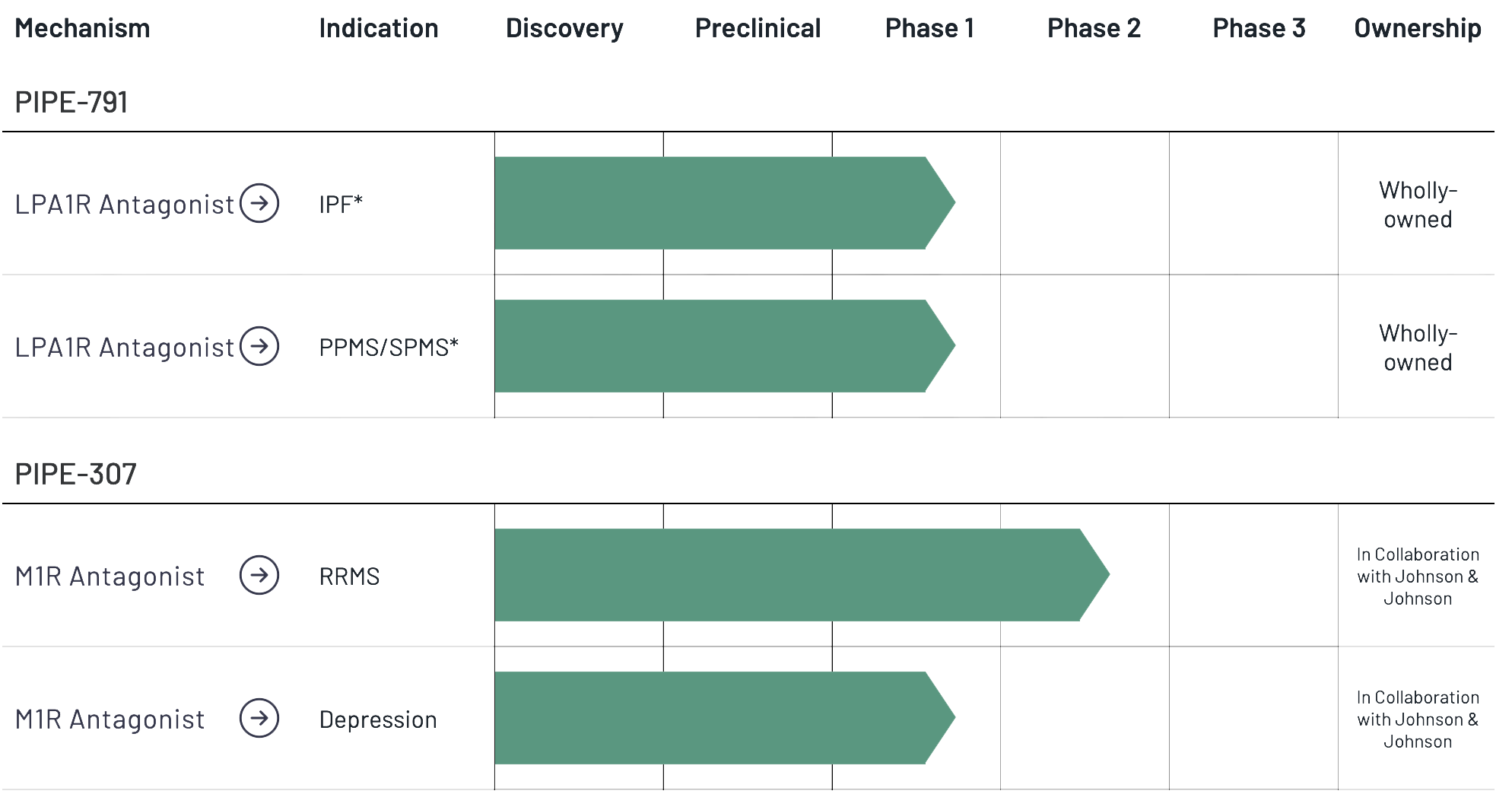
* Single Phase 1 clinical trial of PIPE-791 for the potential treatment of IPF and Progressive MS.
PIPE-791
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (Progressive MS). We are currently conducting a Phase 1 clinical trial of PIPE-791 in healthy volunteers for the potential treatment of IPF. Thereafter, we plan to commence Phase 1b open-label trials in each of IPF and Progressive MS to measure lung and brain receptor occupancy, respectively, by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
LPAIR DC
We are also developing a peripherally-restricted LPA1R antagonist to further expand clinical indications involving LPA1R antagonism. We nominated a development candidate, LPAR1 DC, in December 2023.
PIPE-307
PIPE-307 is a novel, small molecule, selective inhibitor of the muscarinic type 1 M1 receptor (M1R), in development for relapse-remitting multiple sclerosis (RRMS) and depression. We have completed two Phase 1 trials of PIPE-307 in healthy volunteers. We have initiated a Phase 2 trial of PIPE-307 for the potential treatment of RRMS. Contineum is developing PIPE-307 in collaboration with Johnson & Johnson Innovative Medicines (J&J).


